In addition to high school chemistry classes and college entrance exams, have you ever wondered what each chemical element in a periodic table means? Some of them are more obvious, such as oxygen and iron, but the overwhelming majority of people don’t even dream about what becomes the iridium element.
This is just to get an idea of how curious the round trip of swallowing a gram of each element of this table can be. And when asked what would happen to the human organism if all these elements were ingested, the doctor in Public Health from USP and professor of chemistry at Mackenzie University, Rogério Machado, gives a good laugh first. He then explains that “not everything is bad, but it would surely lead to death.”
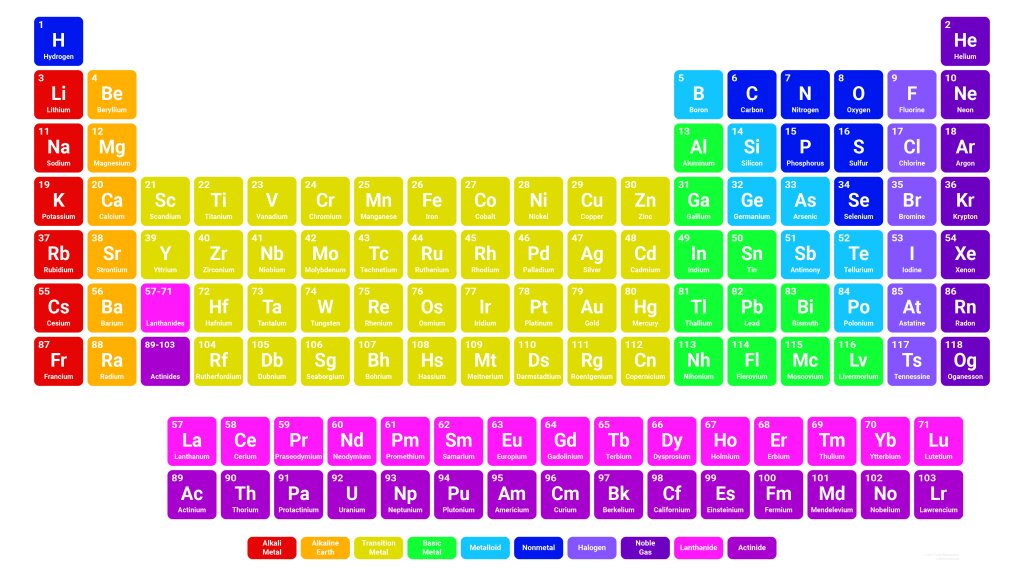
Ah, the periodic table! Of its 119 elements, only the first 92 can be found naturally on Earth. From number 93 onwards, they are considered synthetic or artificial, which means that they can only be obtained through laboratory experiments and are sometimes stable for very few seconds.
For the Mackenzie professor, one of the great challenges of the challenge is to keep the chemical elements isolated: “The element itself you hardly have, because they combine, react. It is very rare to have an isolated atom”. It is the case of metallic sodium, which cannot be exposed to oxygen, which reacts. This element is always dipped in a solvent not to explode. That is, swallowing 1g is already a fatal dose. Goodbye to your head!
Necessary elements for the human body
But before we have extreme reactions, we must first understand the activities of our organism. The human body is basically made up of carbon, hydrogen, oxygen and nitrogen, elements that would not cause harm to the human body if ingested. Of course, for example, everyone is absorbing – or rather breathing – oxygen while reading this article.
Now the elements responsible for the body’s functioning are more varied, such as sodium, potassium, calcium, magnesium, phosphorus, selenium, iron, copper, zinc and many other metals – all necessary for maintaining living and operative organism. For these elements, Professor Machado explains that “after the necessary concentration, they can be toxic, that is, they depend exclusively on their quantity in the organism.”
For example, no more than 1g of sodium and potassium are recommended, which when combined form the bomb of the same name that is responsible for cell transport. Found in sea salt, milk and eggs, sodium, if ingested in large quantities – more than 1g – will be very thirsty and, “if the individual has problems with hypertension, can lead to death.” Meanwhile, the excess Potassium – from legumes such as beans – can cause a shock to the heart, where the organ simply stops.
On the other hand, selenium, which is a necessary micronutrient for all living things – found in bread, fish, meat – is moderately toxic, but 1g is far less than its lethal dose.
Swallowing metals
The professor and chemist explains that “we all need metals in the body, because they turn into ions and each one has its specific function in the body.” But here, the same rule applies: above the necessary quantities, metals can be Very toxic. This is the case with iron. Its absence causes anemia, but in large quantities it is harmful, even if 1g does not lead anyone to death.
The biggest risk is “some metals that humans don’t need to have in their bodies, like mercury”. This is a chemical element that has no function in the body, and how badly it depends on how it is ingested. Fortunately, 1g in solid state should not trigger any kind of reaction, but if you inhale 1g of mercury, you will have a “slow death.”
Among the non-reactive categories are also the materials required for the metal alloys that make up the biomaterials, implants and prostheses, which are mostly metals. Those elements that can be implanted or incorporated into the living organism to restore or replace normally damaged parts. For this reason, it is expected that they will not react in any way in the human body.
To exemplify their behavior in the body, Professor Machado compares them with forks and knives, metals that are in direct contact with the glass, but cannot react in any way, nor oxidize or corrode. For this reason, do not expect any reaction when ingesting 1g of titanium or os, for example. Other solid-state metals that cause no reaction in the body are iridium, platinum, gold and silver.
Ah! The noble gases
In the periodic table there are also the elements in gaseous state. For these, it is worth remembering, according to the chemist Machado, that “in this form, noble gases are inert to the human organism and generally to the environment, that is, they do not react with anything”. Because of this, they are one of the only groups found in isolation in nature.
The challenge to stay alive is that you can’t breathe just them, after all “without oxygen, you will suffocate”. Now imagine that to swallow 1g of Helium (that balloon-filling gas that makes your voice very thin) will be I need to swallow a volume of more than 5L of air. With the other gases it is pretty much the same as neon, argon, krypton, xenon. For now, don’t expect any – dangerous – fun!

Nuclear explosions
After a series of reassuring elements and challenges, one gets to radioactive. At this point, Machado is clear and emphatic: “With radioactivity, they don’t have much to do, my friend. In such cases, 1g is already absurd! Just think what happened to the compound Cesium 137, in Goiania, in 1987, which led to more than 400 people to death, much less than ingestion”.
Like uranium, polonium, and radio, radioactive elements are those capable of emitting radiation — be it alpha, beta, or gamma — spontaneously through their unstable nuclei. “Having contact with any of these elements is, unfortunately, being exposed to radiation with very short wavelengths, which therefore has a high penetration capacity. First of all, they burn and if it does not lead to death, they will trigger cancer formation”.
Also wait for nuclear explosions if these elements reach a concentration of 1g. Like borium or the copernicium, which in 1g, will cause the individual to be destroyed spontaneously with a huge explosion. The ninhonium, of which only a few atoms have been produced so far, is hard to imagine, but wait no less than previous reactions.
Everyday electronics
After exploring different categories of chemical elements, one can also think about the components that make up computers and electronics in general. Even in their reactions in the body, if ingested. Like silicon, which is widely used in chip production and one of the main components of the electronic world, but as an element is practically inert.
Silicon is in electronics, but it is also the raw material of earth and sand, being the second most present element on the planet. This means that it enters and leaves the body without reacting in any way. But Professor Machado remembers that he “can cause you urinary problems, because it does not solubilize. Besides, eating sand is not good for anyone”.
Another unreactive example is copper, which is used in the composition of wires, which would not react in the body and could at most cause vomiting. Although it causes a number of problems, lithium, which is commonly used in batteries and even depression drugs, is also another chemical that should not be deadly.
Arsenic, another semiconductor used in industry in place of silicon, is not so good for the organism because it is very toxic and 1g of it would be enough to lead to death. Due to its great contaminant potential, the element fits well with the term heavy metal, with all its contraindications to human contact.
Experiment Conclusions
There is no other possibility for anyone who resolves to experiment with 1g of each chemical element in the periodic table than a tragic end. And this must happen even before element 100, the fermium, when the body would have been violently exploded and completely destroyed.
But as the professor of chemistry and doctor of public health explained, not everything is absolutely bad, after all many of these substances make up the human organism and others are fundamental to its proper functioning. That’s the maxim: the difference between the medicine and the poison is the dose
Fortunately (or unlucky), a significant portion of the elements mentioned would hardly be found, either because they are rare or because they are produced exclusively in the laboratory. However, the ununennium, known as element 119, could not be found. This is because neither it was discovered and perhaps not even exist. This will require a few more steps of science. But that is a topic for another topic.
Source: With information from Quora